News

21 June 2024
Results of the Annual General Meeting of Shareholders of JSC Irbit Chemical Pharmaceutical Plant. Change of the enterprise's General Director
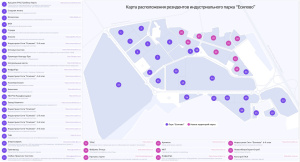
25 April 2024
«SPRING 2024»: first event for residents of the Yesipovo industrial park

4 December 2023
Avexima participates in «CPhI India 2023»




